Neuroblastoma first-line treatment safety | 09.08.22
Research Inquiry
1.1 years old patient with a recent diagnosis of inoperable Neuroblastoma in the left adrenal gland, INRGSS Stage L2, and Opsoclonus-myoclonus syndrome with rapid eye movements and potential decreased visual acuity. Her OMS is treated with dexamethasone PO 20mg/m2/day. The following protocols were suggested as first-line neuroblastoma treatment options according to the European guidelines (low-intermediate risk, group 8): Carboplatin/etoposide ± CADO.
Are the first-line treatments described above safe regarding eye or cognitive impairment, that can be a manifestation of chronic OMS?
This summarized report will review the possible linkage between accepted first-line Neuroblastoma treatments and eye damage or cognitive impairment.
Conclusion
According to our review of the latest clinical trials and literature, there is no association between the accepted first-line Neuroblastoma treatments and eye damage or cognitive impairment. There are inquiry-related adverse events that are mentioned on some drug labels, but the evidence is based mainly on post-marketing data that can’t be quantified. As a result, we can advise starting treatment together with a routine evaluation of the patient’s eyes and mental state.
Research Findings
- CADO protocol is a combination of cyclophosphamide IV and doxorubicin IV As so, we will review the latest literature about CADO combination safety, and present each drug safety brochure as declared by the manufacturer.
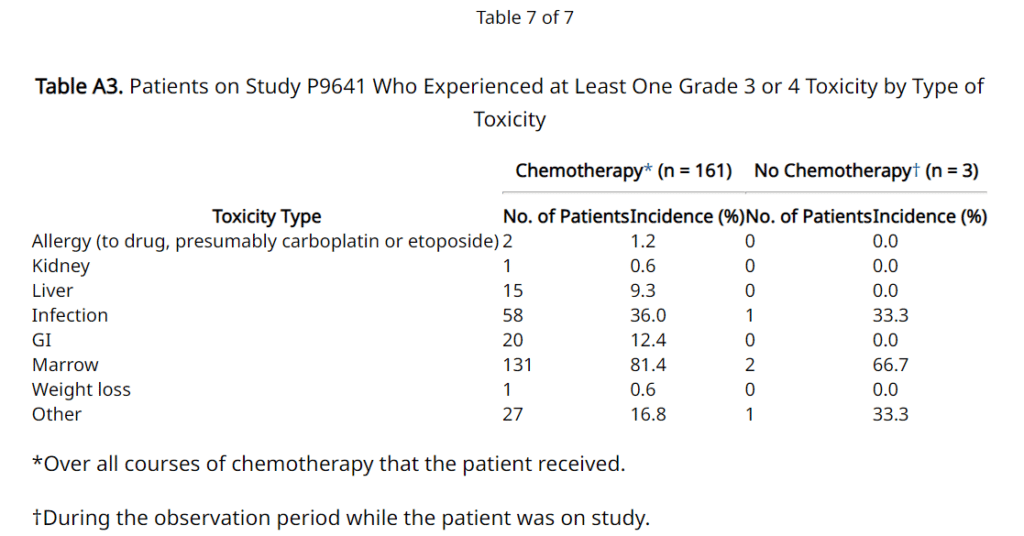
- Research (Children’s Oncology Group Study P9641) published in the Journal of Clinical Oncology in 20121 measured the outcome after surgery alone or with restricted use of chemotherapy (carboplatin, cyclophosphamide, doxorubicin hydrochloride, etoposide) for patients with low-risk neuroblastoma. The main adverse events mentioned by the researchers were related to Marrow (81.4%), Infection (36%), and GI (16.8%). No sight damage or cognitive impairment adverse events were reported under “Other” section in the table.
- A phase III clinical trial2 that was later published in the Journal of Clinical Oncology in 20193 tested combination chemotherapy (carboplatin, cyclophosphamide, doxorubicin hydrochloride, etoposide, filgrastim) and surgery with or without isotretinoin in treating 464 young patients with Neuroblastoma. The main adverse events mentioned by the researchers were related to Anemia (301 events), Neutropenia (73 events). There were only 2 eye-related adverse events (one infection and one unspecified), and one Cognitive disturbance event – both were not serious.
- An Article published in The New England Journal of Medicine in 20104 researched the outcomes of reduced chemotherapy (carboplatin, etoposide, cyclophosphamide, and doxorubicin) for intermediate-risk neuroblastoma on 470 patients, in a phase 3, nonrandomized trial. The main grade 3 or 4 toxic effects mentioned by the researchers were related to myelosuppression (68%). Infections (11%) Heart (4%), Kidneys (2%), and hearing (<1%). No sight damage or cognitive impairment adverse events were reported.
- Pfizer’s Carboplatin label5 mentions a long list of side effects that are linked to Carboplatin. Two of the inquired side effects are mentioned in the brochure:
- Vision problems: temporary worsening of eyesight or changes to your vision, temporary loss of sight.
- Encephalopathy (mental changes): symptoms may include changes in memory, difficulty focusing, change in personality, fatigue, and progressive loss of consciousness.
It is worth mentioning that Pfizer placed it under “Unknown frequency” section, which is dedicated to rare side effects.
- Baxter’s Cyclophosphamide FDA label6 summed a few systems that are prone to adverse reactions, like the Hematopoietic, Gastrointestinal, and skin systems. Postmarketing experience mentions visual impairment, conjunctivitis, and lacrimation, but because they are reported from a population of unknown size, precise estimates of frequency cannot be made.
- Baxter’s Etoposide Phosphate FDA label7 describes a few serious adverse reactions that can happen while using the drug, like Myelosuppression, Secondary leukemias, and Hypersensitivity reactions. Transient cortical blindness and optic neuritis are mentioned in the “other” toxicities section.
- Pfizer’s Doxorubicin label8 mentions a list of adverse events that are linked to the drug, like vomiting, alopecia, and leukopenia. Postmarketing experience mentions conjunctivitis, keratitis, and lacrimation, but because they are reported from a population of unknown size, precise estimates of frequency cannot be made.
References
- https://pubmed.ncbi.nlm.nih.gov
- https://clinicaltrials.gov
- https://www.ncbi.nlm.nih.gov
- https://pubmed.ncbi.nlm.nih.gov
- https://www.pfizer.ca/sites/default
- https://www.accessdata.fda.gov
- https://www.accessdata.fda.gov
- https://labeling.pfizer.com