CAR T-Cells Therapy in T-cell ALL | 09.08.22
Conclusion
According to our review of the latest clinical trials and literature, although CAR T-cell treatment has promising in vivo and in vitro results, using it for T-ALL first relapse is debatable, considering the lack of phase II clinical trial efficacy data, risk of toxicity (as described in the research, and in the 2018 ASTCT Consensus mentioned below), and other suggested treatments that are recommended by the NCCN and NIH guidelines.
Research Inquiry
a 14-year-old boy who was diagnosed 4 years ago with T-Cell ALL (Cervical LN + molecular involvement of BM). The patient received chemotherapy according to EURO LB 02321 with a very good response. In the last follow-up, he was diagnosed with Relapsed T-Cell ALL. BM relapsed by 75% lymphoblasts (lab results are attached to the inquiry web form).
Is CAR T-Cells Therapy effective and safe enough to be considered as a second-line treatment for T-Cell ALL?
This summarized report will review the efficacy and safety of CAR T-Cells Therapy in T-cell ALL as described in the latest literature and clinical trials, in comparison to other T-cell ALL second-line treatments.
Meta Medical Findings
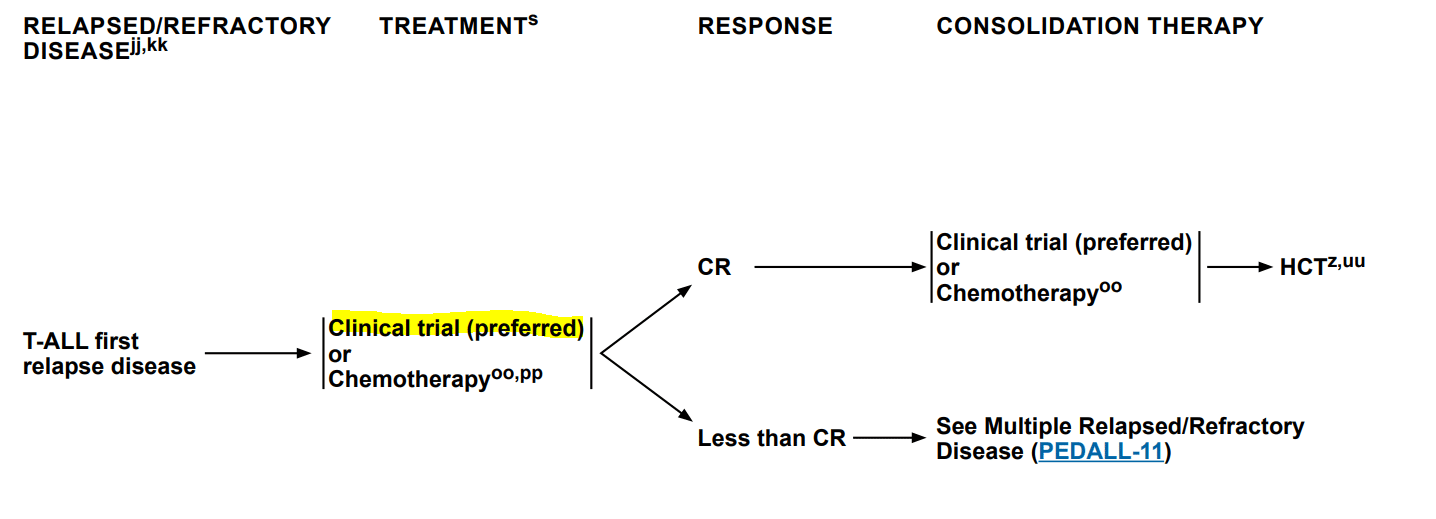
According to the 2022 NCCN Clinical Practice Guidelines2 for Pediatric Acute Lymphoblastic Leukemia, the recommended way of action in T-ALL first relapse is to join a clinical trial (considered the preferred route by NCCN) or to start chemotherapy. The guidelines suggest using Nelarabine, a nucleoside analog that is currently approved for the treatment of patients with T-ALL who have relapsed disease after at least two chemotherapy regimens, that showed a 55% response rate among the subgroup with T-ALL and first bone marrow relapse. CAR T-cell therapy is not mentioned in the guidelines.
The updated 2022 NIH Cancer guidelines4 [b][c]for Childhood Acute Lymphoblastic Leukemia Treatment highlights that patients with relapsed T-ALL have much lower rates of achieving a second complete response with standard reinduction regimens than do patients with B-cell phenotype. As a result, the writers suggest three main treatment options (CAR T-cell therapy is not mentioned in the guidelines):
- Using the T-cell selective agent, Nelarabine, which resulted in response rates of approximately 50%.
- Using the combination of Nelarabine, Cyclophosphamide, and Etoposide, which has also been used in patients with relapsed/refractory T-ALL.
- Using the combination of bortezomib plus vincristine, prednisone, pegaspargase, and doxorubicin resulted in a second CR rate of 68% in T-ALL patients in the first relapse.
An article published in the International Journal of Molecular Sciences in 20214 reviewed some New Therapeutic Strategies in Pediatric T-Cell Acute Lymphoblastic Leukemia. According to the writers, despite the great development of targeted cellular therapies, T-lineage neoplasms remain a challenge for CAR-T cells due to the limited ability to distinguish between therapeutic, normal, and malignant T-cells.The writers mention a list of positive pre-clinical results and one phase I CD5-targeted CAR-T cells for patients with T-cell malignancies but alsohighlight the risk of toxicity (destruction of normal T-cells, leading to life-threatening opportunistic infections) and the lack of clinical evidence that supports using the therapy.
The risk of toxicity is also supported by the 20185 ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells, which mentioned that Cytokine release syndrome (CRS) and neurotoxicity are common after CAR-T treatment.
There are several articles that were published in the last years regarding CAR T-cell therapy for T cell hematologic malignancies:
| Journal | Publish year | Model | Summary |
| Blood journal6 | 2022 | In vivo (animal study) | The article reviewed CD7 CAR T-cell for the Immunotherapy of Hematological malignancies, with a T-ALL focus. The writers showed (in vitro) that CD7 T-cells derived from naturally occurring CD7 negative T cells have promising effector function, as judged by their ability to expand, persist, and elicit potent antitumor activity against CD7+ T-ALL and CD19+ B-ALL. |
| Journal of Clinical Oncology7 | 2020 | Phase I Clinical trial | CD7 CAR-T on adults diagnosed with r/r T-ALL showed an efficacy rate of 80% OR (4/5). |
| Blood journal8 | 2019 | Phase I Clinical trial | The article described a Phase I dose escalation study (NCT03081910) of autologous CD5-directed chimeric antigen receptor T cell (CD5 CAR T) therapy for relapsed or refractory T-cell leukemia and lymphoma in 9 patients (8 adults and 1 adolescent; age 16-71 years [median 62 yrs]). Complete responses (CR) were achieved in 3 patients, one with angioimmunoblastic T cell lymphoma (AITL), one with peripheral T cell lymphoma (PTCL), and one with T-ALL (out of 4). Two of these patients did not wish or were unable to proceed to planned HSCT and relapsed with their underlying CD5+ malignancy at 6 weeks and 7 months post-infusion. |
| Blood journal9 | 2017 | In vitro and in vivo (animal study) | The writers demonstrated that CD7 CAR T cells have robust activity against T-cell malignancies in vitro and in vivo. |
| Nature Leukemia10 | 2015 | In vitro and in vivo (animal study) | Researchers have designed a CAR, CD4CAR, which redirects the antigen specificity of CD8+ cytotoxic T cells to CD4-expressing cells, and showed that CD4CAR-expressing CD8+ T cells are efficacious in ablating malignant CD4+ populations, with potential use as a bridge to transplant or stand-alone therapy for the treatment of T-ALL. |
- A phase 1 clinical trial evaluating the efficacy and safety of CD7 CAR-T cells reported results on 3 pediatric patients diagnosed with T-ALL/LBL7:
| Patient | Diagnosis | Result | Side effects |
| 1 | Refractory ALL with myeloid differentiation – received intensive chemotherapy and allogeneic HSCT. | 1. Abnormal cells in BM decreased from 70.03% to 19.57%. 2. 5 Month OS,3 month PFS. |
Grade 3 CRS |
| 2 | ALL (T/B mixed type) but relapsed with CNS involvement. received radiotherapy in addition to intensive chemotherapy. | 1. MRD negative CR(day 28). 2. Significant CAR-T expansion. 3. Was still in remission after 2 months. |
Grade 3 CRS |
| 3 | Stage VI of T-LBL, which recurred after multi-cycle chemotherapy (BFM-90) and autologous HSCT. | 1. MRD negative CR (day28). 2. Significant CAR-T expansion. 3. Was still in remission after 1 month. |
Grade 3 CRS |
- Several CAR-T therapies for T-Cell Acute Lymphoblastic Leukemia clinical trials are open and recruiting, mainly in China and the United States. Most of them focus on CD7 CAR T-cells and are relevant to the age of the patient [11,12,13,14].
References
- https://www.ncbi.nlm.nih.gov
- https://www.nccn.org
- https://www.cancer.gov
- https://www.ncbi.nlm.nih.gov
- https://pubmed.ncbi.nlm.nih.gov
- https://ashpublications.org
- https://ascopubs.org
- https://sciencedirect-com.ezlibrary.technion.ac.il
- https://ashpublications.org
- https://www.nature.com
- https://clinicaltrials.gov
- https://clinicaltrials.gov
- https://clinicaltrials.gov
- https://clinicaltrials.gov